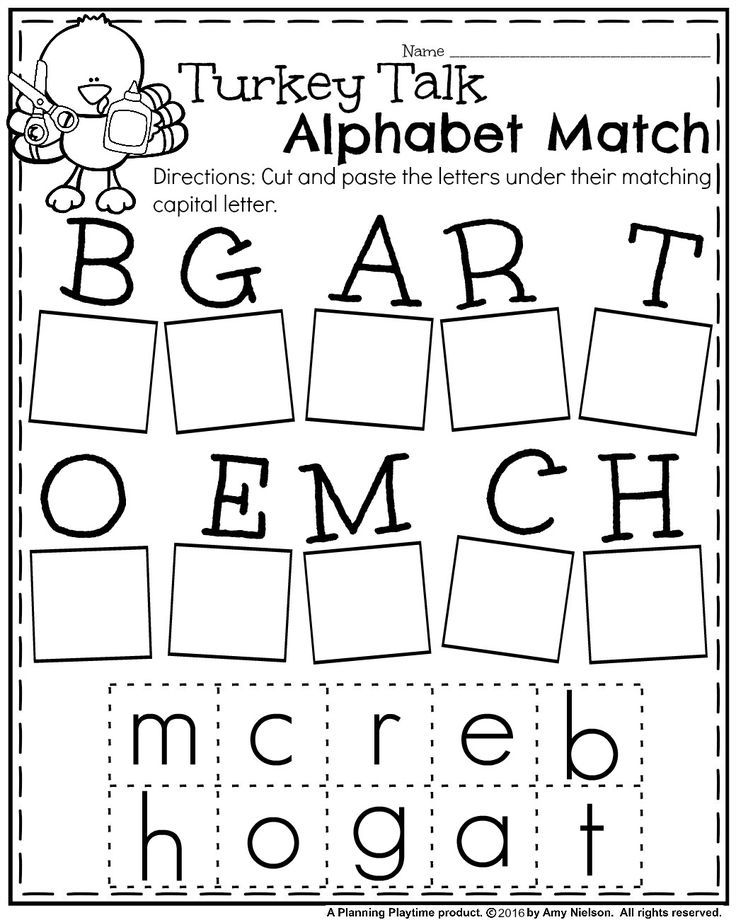
The legislative branch congress the executive branch president and the judicial branch supreme court. Identify the branch doing the checking and the branch being checked.
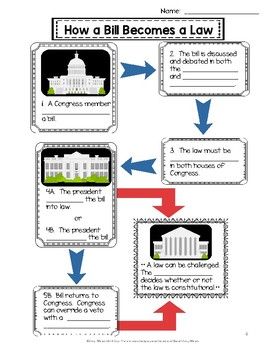
U S Government Civics Freebie 3 Branches Checks Balances Bill Becomes Law Social Studies Worksheets Co Teaching Teaching Social Studies
In order to make sure that one branch didn t become too powerful the constitution has checks and balances that enable each branch to keep the.

Checks and balances worksheet. More than one answer is possible per section. Experts say it s a particularly heated showdown involving america s system of checks and balances. The constitution of the united states established the three branches of the united states government.
Checks and balances is a political principle which describes how the branches of government work with each other. In many cases the system works without much fuss as when. A worksheet various governmental powers are listed below.
Founded by justice sandra day o connor out of her concern that students are not getting the information and tools they need for civic participation and that civics teachers need better materials and support. About this quiz worksheet these learning tools will help you quickly assess your understanding of the system of checks and balances in the u s. This social studies worksheet designed for a fourth and fifth grade curriculum introduces children to checks and balances in the united states government along with how this system is used to keep any one branch from taking too much power.
Start studying worksheet on checks and balances. The checks and balances system. Checks and balances the constitution created three separate branches of the government.
More than one answer is possible per section. The framers spread power among the three federal branches executive legislative and judicial and gave each branch the ability to curb or check the power of the other two. The checks and balances system.
A worksheet various governmental powers are listed below. Learn vocabulary terms and more with flashcards games and other study tools. Web based education project designed to teach students civics and inspire them to be active participants in u s.
These branches are the executive legislative and judicial. You will be quizzed on how the system. Identify the branch doing the checking and the branch being checked.

Checks And Balances Practice Worksheets Check And Balance Practices Worksheets Homework Worksheets

This Worksheet Matches My Interactive Powerpoint Presentation Which Describes Various Che Teaching Social Studies Teaching History Social Studies Middle School

Checks And Balances System Worksheet Education Com Teaching Government Social Studies Worksheets Social Studies Elementary

Checks And Balances Worksheets Check And Balance Teaching Government Social Studies Education

Government Checks And Balances Checks And Balances Chart Powerpoint Teaching Government Government Lessons 3rd Grade Social Studies

Checks And Balances Worksheet Answers You Better Check Yourself Separation In 2020 Check And Balance Simplifying Rational Expressions Simplifying Algebraic Expressions

Lesson Plan Sos Teachers 6th Grade Social Studies 3rd Grade Social Studies Social Studies Teacher

Checks And Balances Worksheet Google Search Check And Balance Worksheets Balance

Checks And Balances Check And Balance Government Lessons Learning Stations

Checks And Balances Worksheet Answers Beautiful Checks And Balances Worksheet Funresearcher In 2020 Check And Balance Volume Worksheets Worksheets

4th 5th Grade History Learning Activity Checks And Balances Learning Liftoff Social Studies Education Teaching Social Studies Social Studies Class

Checks And Balances Worksheets Check And Balance Worksheets Worksheet Template

Checks And Balances Worksheet Answers New Checks And Balances Worksheet In 2020 Check And Balance Worksheets Worksheet Template

3 Branches Of Government Checks Balances Check And Balance Government Social Studies Branches Of Government

Checks And Balances Graphic Visual Summary Explains Our System Of Checks And Balances Social Studies Education Social Studies Centers Teaching Social Studies

Worksheet Executive Branch Of Government Social Studies Worksheets Teaching Government Social Studies Elementary

Check The Power Checks And Balances Va Civics Economics Sol Ce 6b Check And Balance Power Social Studies Middle School

Checks And Balances Worksheet Answers Elegant Danielle Keane Teaching Resources In 2020 Check And Balance Worksheets Worksheet Maker

The Checks And Balances System Aw Orksheet Check And Balance Simplifying Algebraic Expressions Simplifying Rational Expressions